Drs. Bansal and Dahia Bring a Global Pragmatic Trial to UHS and VA Patients
Contributor
March 8, 2019

One of the strategic goals of the Clinical and Translational Science Award (CTSA) Program sponsored by the National Institutes of Health (NIH) is to improve the health of the communities we serve.
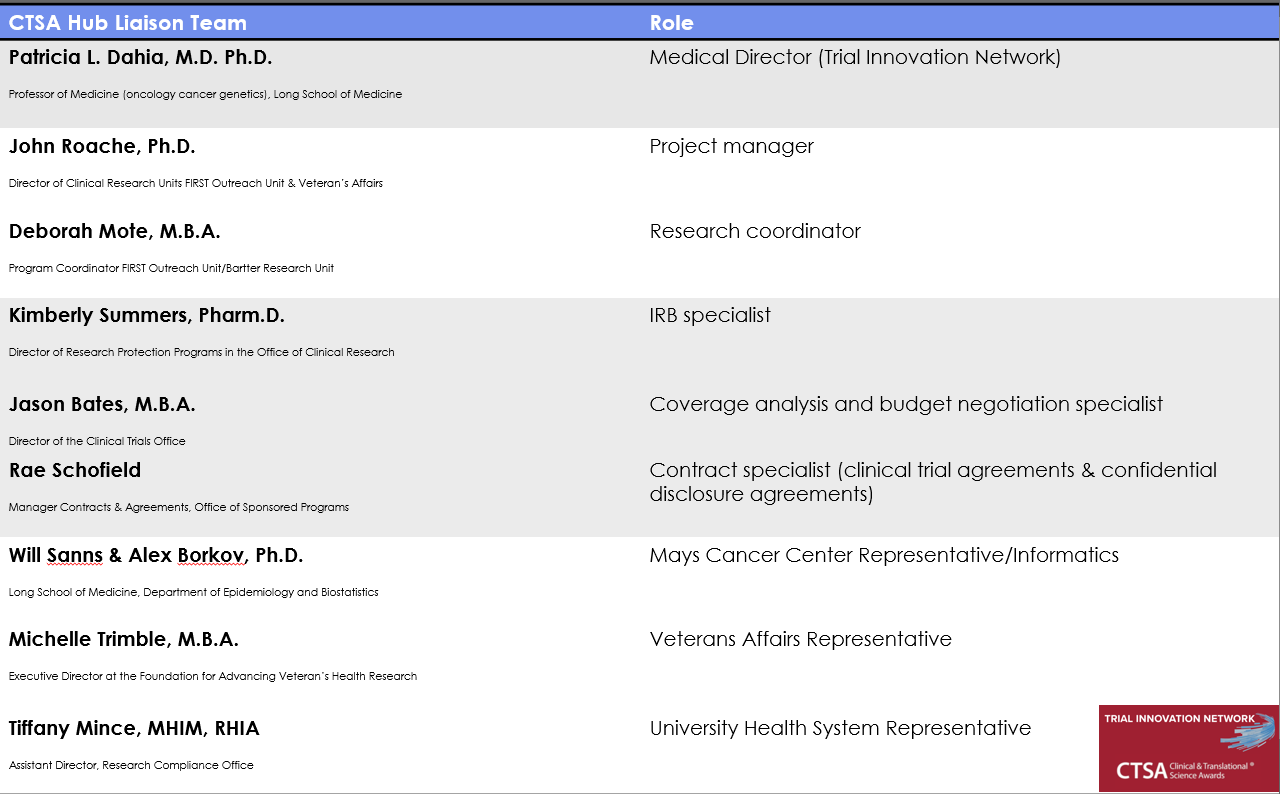
For over a decade, UT Health San Antonio has served as a CTSA hub, giving our investigators access to a nationwide CTSA network, which includes more than 50 academic medical centers and a wealth of curated resources and expertise. Patricia Dahia, M.D., Ph.D., professor of medicine (hematology-oncology) and medical director for the Trial Innovation Network Hub Liaison Team at UT Health San Antonio, is excited about the Trial Innovation Network (TIN), a collaborative initiative from the NIH that leverages the resources and expertise of CTSA sites to improve the quality and efficiency of clinical trials.
Shweta Bansal, M.D., associate professor of medicine (nephrology), is the first faculty member from our institution to be approved as a TIN site principal investigator. This innovative clinical trial, SPIRRIT, studies the effect of spironolactone in Swedish and United States patient populations suffering from heart failure with preserved ejection fraction (HFpEF). It is a multisite, pragmatic trial sponsored by the NIH and coordinated by the Duke Clinical Research Institute, a Trial Innovation Center (TIC). Dr. Bansal, a zealous nephrologist who has conducted numerous industry and federally sponsored clinical trials, immediately agreed to be part of it when she was contacted by Bob Clark, M.D., IIMS Director and the CTSA principal investigator. Dr. Bansal views the study as an opportunity to help her chronic kidney disease patients, many of whom have HFpEF, while advancing her own clinical and research interest in spironolactone as a treatment option for this population.

Dr. Dahia (left) consults with Dr. Bansal.
“I am very excited about this opportunity and responsibility bestowed on me to advance the science with generic medications like spironolactone, which has great potential to help our cardiorenal patients, especially those who have difficulty accessing costlier brand-name medications,” Dr. Bansal shared.
As the site investigator, Dr. Bansal will enroll 15-16 patients in SPIRRIT. Being part of this study allows her to collaborate with distinguished national and international colleagues who pioneered one of the largest clinical trials, TOPCAT, studying the effects of spironolactone in HFpEF. She also expressed excitement at conducting her first pragmatic trial wherein the intervention is tested in a broad routine clinical practice, rather than in a well-defined controlled setting. This real-world setting mitigates the challenges associated with traditional clinical trials that may not lead to generalizable results.
“Operationally efficient multisite clinical trials can accelerate the translation of novel clinical discoveries to patient care,” said Dr. Dahia. “We hope that our investigators will avail themselves of the invaluable resources offered by the TIN.”
Services provided by TIN, such as centralized IRB, master contracting agreements, advice on study design, and approaches to improve patient recruitment and engagement, can streamline the process of conducting clinical trials. Capitalizing on TIN resources, Dr. Bansal’s participation in this trial will give her the ability to bring cost-effective treatment options to cardiorenal patients in San Antonio. Through the CTSA’s TIN, these patient benefits can be disseminated to many other centers across the nation.